Citation
Puerta‐Falla, G.; Balonis, M.; Le Saout, G.; Falzone, G.; Zhang, C.; Neithalath, N.; Sant, G. Journal of the American Ceramic Society 2015, 98(12), 4076-4089.
Puerta‐Falla, G.; Balonis, M.; Le Saout, G.; Falzone, G.; Zhang, C.; Neithalath, N.; Sant, G. Journal of the American Ceramic Society 2015, 98(12), 4076-4089.
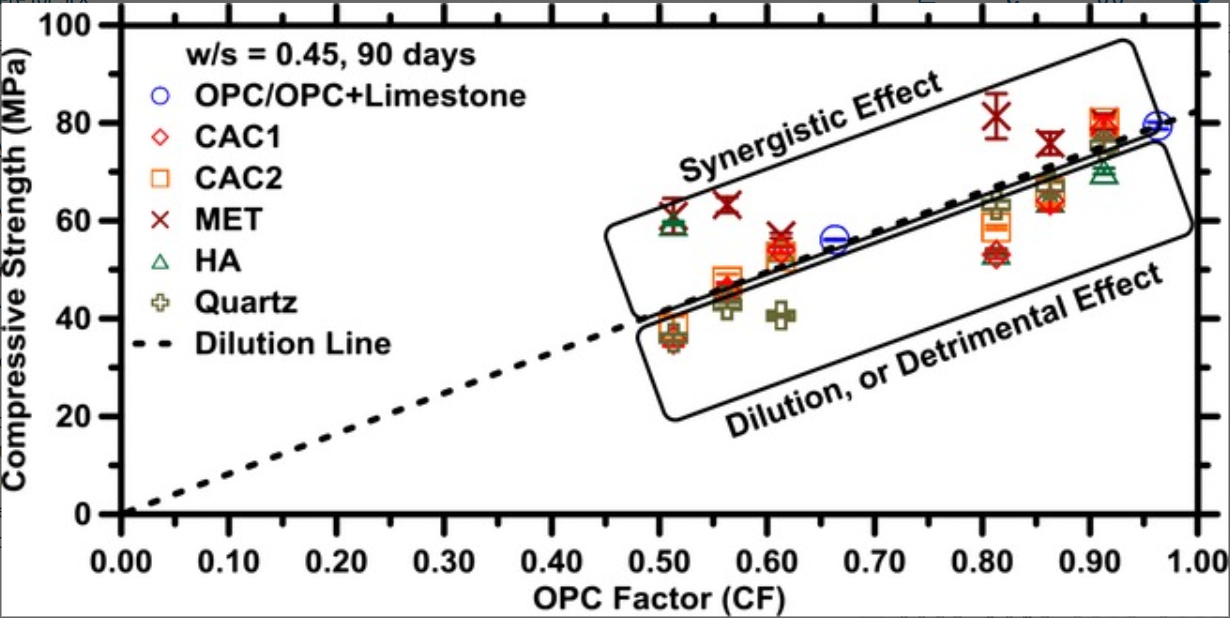
When limestone (CaCO3) is present in ordinary portland cement (OPC), carbonate‐AFm phases (i.e., hemi‐ and/or mono‐carboaluminate) are stabilized at the expense of the sulfate‐AFm, which is more commonly found in cement systems. In OPC, the quantity of AFm hydrates formed is often limited by the availability of aluminum. Therefore, as a means of enhancing AFm phase formation, this study elucidates the role of aluminous sources including: calcium aluminate cements, metakaolin, and a hydratable alumina to determine if their addition would enhance limestone reactions and carbonate‐AFm formation in cement systems. The results of a detailed study including: X‐ray diffraction, strength measurements, thermogravimetric analysis, and thermodynamic calculations are used to quantify solid phase constitutions, and the extent of limestone reacted. The results suggest that, the amount of limestone reacted and the specific carbonate‐AFm formed is sensitive to both, the nature of the aluminous source and limestone content. Pozzolanic reactions which occur when metakaolin is used as an aluminous source are noted to be especially beneficial in offsetting the effects of OPC replacement. It is noted that although the different aluminous materials react with different quantities of CaCO3during hydration, enhanced carbonate‐AFm formation alone is insufficient to ensure strength equivalence, when OPC is replaced by limestone.