Citation
Falzone, G.; Balonis, M.; Sant, G. Cement and Concrete Research 2015, 72, 54-68.
Falzone, G.; Balonis, M.; Sant, G. Cement and Concrete Research 2015, 72, 54-68.
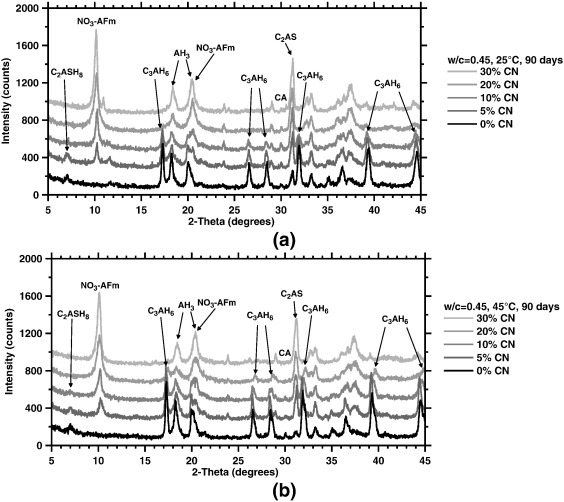
Phase conversion phenomena are often observed in calcium aluminate cements (CACs), when the water-rich hydrates (e.g., CAH10, C2AH8) formed at early ages, at temperatures ≤ 30 °C, expel water in time to form more compact, less water-rich structures (C3AH6). The phase conversions follow a path regulated by the thermodynamic stabilities (solubilities) of phases. Based on this premise, it is proposed that conversion phenomena in CACs can be bypassed by provoking the precipitation of phases more preferred than those typically encountered along the conversion pathway. Therefore, X-AFm formation (where in this case, X = NO3−) triggered by the sequential addition of calcium nitrate (Ca(NO3)2 = CN) additives is identified as a new means of bypassing conversion. A multi-method approach comprising X-ray diffraction (XRD), thermal analytics, and evaluations of the compressive strength is applied to correlate phase balances and properties of CAC systems cured at 25 °C and 45 °C. The results highlight the absence of the C3AH6 phase across all systems and the curing conditions considered, with enhanced strengths being noted, when sufficient quantities of CN are added. The experimental outcomes are supported by insights gained from thermodynamic calculations which highlight thermodynamic selectivity as a means of regulating and controlling the evolutions of solid phase balances using inorganic salts in CACs, and more generally in cementing material systems.